Electrolytes - Water Balance and Hydration
Athletes hear a lot about electrolytes, but what exactly are these obscure little entities?
ELECTROLYTES
Tiny but mighty, these electrically charged minerals are essential to life.
In science the definition of the word is often in the name electro- (electricity) and -lyte (loosen). When dissolved in water, electrolytes loosen and separate into individual atoms that carry an electrical charge. The charge allows them to conduct electricity in water.
Electrolytes perform many functions in the body, but today’s article will focus on their role in water balance/hydration.
Electrolytes are Elements. Important electrolytes in the body include sodium (Na+), potassium (K+), magnesium (Mg2+), chloride (Cl-), and calcium (Ca2+).
FOOD SOURCES OF ELECTROLYTES
You can meet all your electrolyte needs from foods. Whole foods, including fruits, vegetables, nuts, dairy and grains, are excellent sources of potassium, magnesium, chloride, and calcium. Packaged foods and restaurant meals/items can be very high in sodium (e.g. 1 slice of cheese pizza > 500mg sodium). Breads, cheese, and milk also contain high amounts of sodium.
FUNCTIONS OF ELECTROLYTES
Electrolytes perform a variety of vital functions in the body:
Transmit nerve signals
Enable muscle contraction
Maintain water balance
In this article we’ll focus on sodium and how electrolytes play a role in water balance and hydration.
ELECTROLYTES IN WATER BALANCE
Electrolytes, especially sodium, have three important functions:
Keep water in place
Bring water into the body
Hold water in the body
ELECTROLYTES KEEP WATER IN PLACE
The body is ~65% water. Thankfully, that water doesn’t just slosh around inside of us like a big water balloon, rather it’s stored in different compartments, intracellular - inside our cells or extracelluar - outside the cells (e.g. in the blood).
Water balance between compartments is essential to life. Too much water inside the cells and they could swell, loose function, or burst. Not enough water in the blood vessels and the heart can’t efficiently deliver nutrients to the body and filter wastes away.
Keeping water where it belongs is tricky business because water can flow freely through cell walls. So how does the body get water to behave?
Electrolytes! With all their allure and attraction, electrolytes beckon and corral water in place.
How? Through a process called osmosis. Osmosis is the passive pull and flow of water through a cell membrane. Water flows from an environment with a lower concentration to a higher concentration of dissolved particles (see image below).
Osmosis in Action: The experiment begins with two equal compartments of water separated by a semi-permeable membrane (the membrane allows water to pass but not sodium). Salt is added to one side. Water is pulled towards the more concentrated solution and drawn across the semipermeable membrane.
Think of water like a lost puppy. Where sodium goes, water follows. Knowing water doesn’t like being left behind, the body works to strategically place sodium where water needs to be - i.e. in the blood. Sodium’s presence in the blood attracts water, which is a crucial factor for maintaining blood volume. Adequate blood volume and pressure ensures the heart can deliver oxygen and nutrients throughout the body.
ELECTROLYTES BRING WATER INTO THE BODY
Electrolytes not only keep water in the right place, but also help shuttle the water you drink into the body.
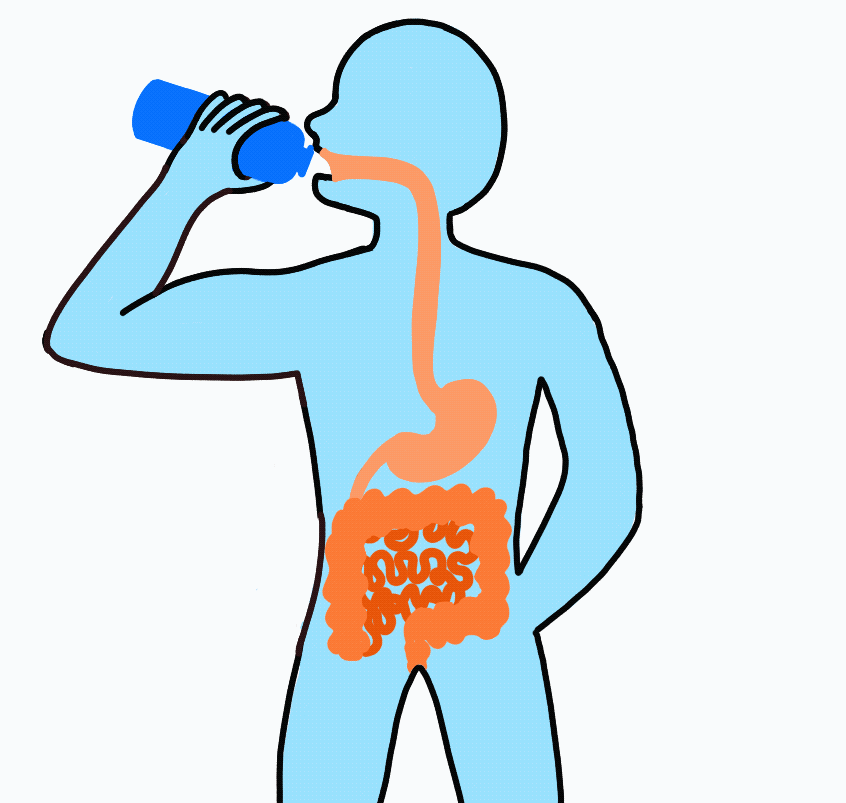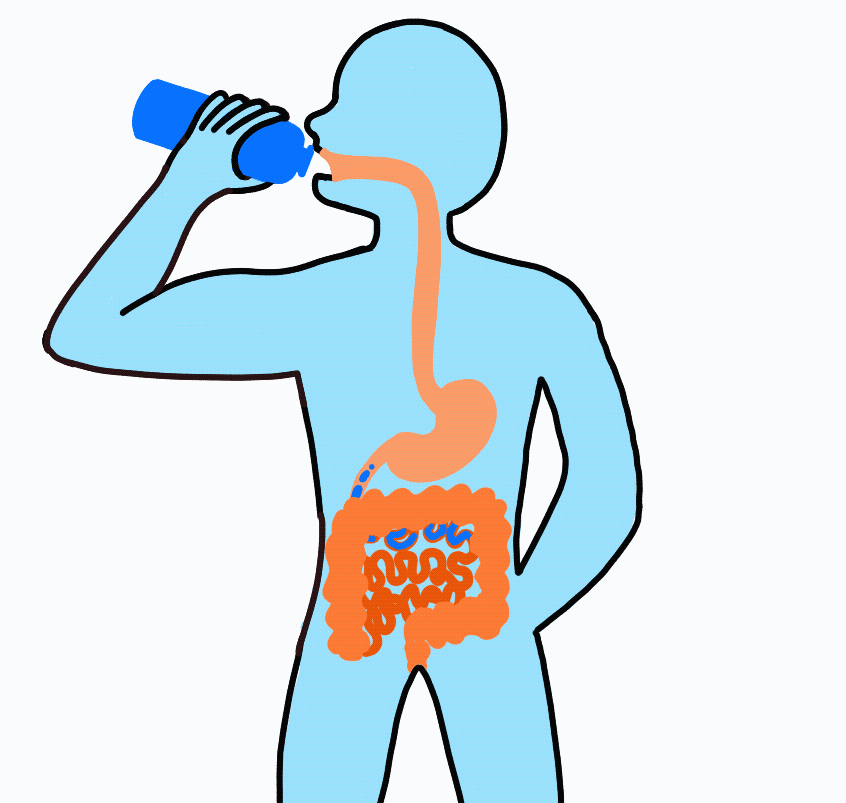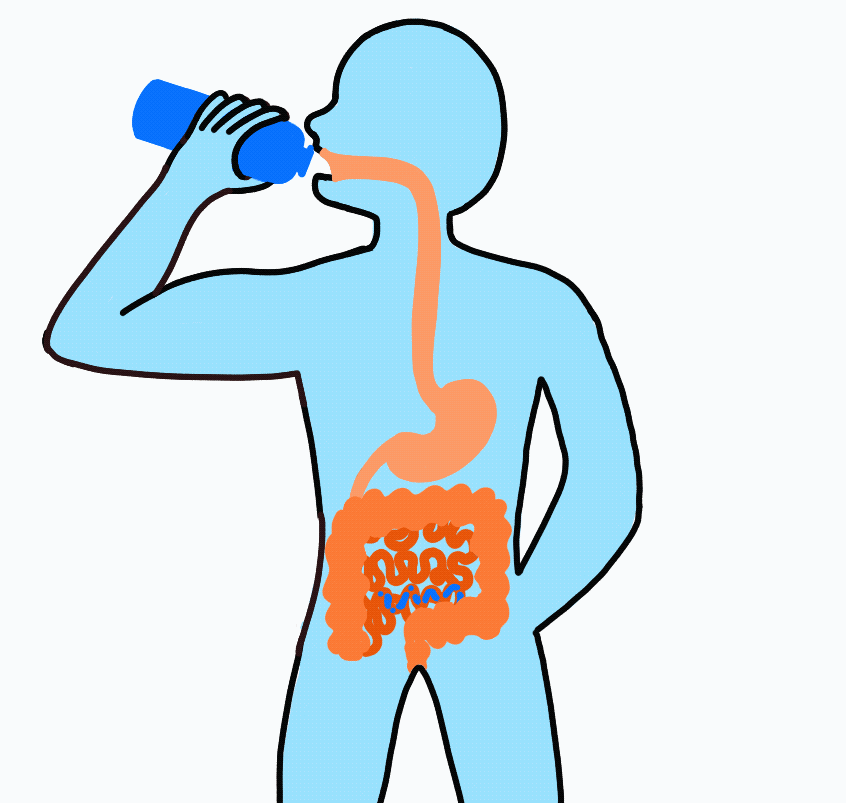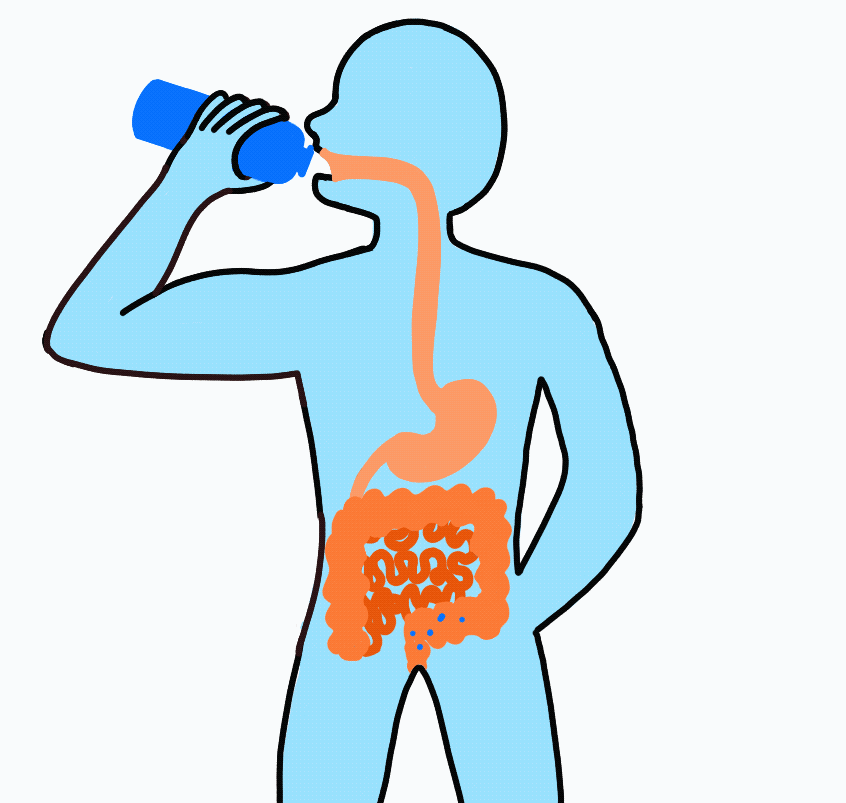
Imagine drinking a sports drink containing water, electrolytes, and carbohydrates. The liquid enters your mouth then passes through the esophagus on its way to the stomach. After sloshing around in the stomach for a short amount of time, it flows on to the small intestine.
At this point, even though swallowed, the sports drink is still considered outside the body. To be inside the body, the nutrients must be absorbed across the intestinal wall and enter the blood. Because water follows sodium, when sodium and carbohydrate are transported across the intestinal wall water will follow.
Nutrient Absorption: Carbohydrate, sodium and water are absorbed across the lining of the intestine and into the blood stream. The absorption of sodium and carbohydrate increase the concentration inside the intestinal cell (enterocyte) and water passively moves across.
ELECTROLYTES HOLD WATER IN THE BODY
Rehydrating after an intense workout is an important part of recovery, especially when it’s hot outside. To learn more about dehydration and its negative effects check out my first post on hydration here. For optimal rehydration AVOID THE INSTINCT TO CHUG. Drinking too much too fast can have a dehydrating effect. A slow and steady drinking pattern is key.
How can drinking water be dehydrating? Water in the intestines has a lower concentration than the contents inside the intestinal cells, which means water will be quickly absorbed into the blood stream (see osmosis above). This causes a rapid rise in blood volume and dilutes the concentration of the blood. These changes are detected by brain and kidneys, which respond by increasing urine production and decreasing your drive to drink. The end result, the water you just drank to replace what you lost during exercise will be removed as pee.
How do you avoid this volume response? Electrolytes, especially sodium, from food or beverages can help you hold on to the water you drank. Why?
1) Consuming sodium with water post workout will prevent the dilution (or drop in the concentration) of the blood and inhibit the brain from thinking you are over-hydrated. The brain and kidneys are satisfied because the electrolytes in the blood are at normal levels and see no need to remove fluid from the body.
2) Sodium increases thirst, which motivates you to keep drinking - remember a slow and steady pace or a sip don’t chug approach is best.
DO YOU NEED AN ELECTROLYTE DRINK?
For general hydration, no, most people do not need to add electrolytes in their diet. The foods we eat provide plenty of electrolytes.
However, for post exercise recovery, replacing fluid AND electrolytes is recommended when the workout lasts more than an hour or takes place in hot or humid conditions. Food post workout provides electrolytes and energy to replenish glycogen and fuel for growth and repair (see sources above), but if you don’t have the option to eat right away an electrolyte drink can be a good choice.
WHICH ELECTROLYTE DRINK IS RIGHT FOR YOU?
Electrolyte drinks are popular and widely available. Check out this great review of popular electrolyte drinks from Eleat Sports Nutrition here. For young athletes the best brands have limited ingredients with no caffeine (AVOID drinks with ENERGY on the label, these contain caffeine. But always read labels when trying something new the beverage could still contain caffeine even if it is not labeled Energy.)
Studies have tested various post-exercise hydration strategies and the winner turns out to consistently be water, plus electrolytes (sodium is the major player for hydration with potassium showing a possible mild effect), plus carbohydrates. For post-exercise recovery carbs provide two benefits: 1) glucose to replenish glycogen stores used during exercise and 2) improved water absorption and retention.
Below is a list of some of most common, easy to find products and their nutrition profile.
1 LMNT and Liquid IV have very high levels of sodium and are best suited for ultra endurance athletes
2 Coconut water has a much lower sodium content than any of the beverages.
RECIPES
And why not try making your own electrolyte drink with these super simple recipes!
Coconut Electrolyte Drink - Mix
1/8 tsp salt (295mg sodium)
1/2 cup coconut water (200mg potassium and 30mg sodium)
3 tsp sugar (12g carbs)
Pineapple juice splash (4g carbs and 40mg potassium)
1 1/2 cups water
Citrus Electrolyte Drink - Mix
1/8 tsp salt (295mg sodium)
4 tsp sugar (16g)
Fresh squeeze of lemon/lime/orange juice (30g /(30g /60mg potassium, respectively)
2 cups water
SUMMARY
To sum up the story of electrolytes and hydration, remember the following:
Electrolytes are minerals that separate in water and carry a charge
Sodium is the most important electrolyte for hydration/water balance
Sodium attracts water; water follows sodium through the process of osmosis
The important functions of sodium are to keep water where it belongs (i.e. in the right compartments), bring water into the body, and hold water in the body
You can meet all your electrolyte needs through food
SIP DON’T CHUG - Consume electrolytes post workout if more than 60 minutes and/or in hot humid conditions and
The best post exercise drink for hydration includes water, sodium and carbohydrate
Read labels on electrolyte products and avoid drinks with caffeine or labeled “Energy”
And be sure to check out my previous article Beat the Heat Hydration!
REFERENCES
Osmolality of Commercially Available Oral Rehydration Solutions: Impact of Brand, Storage Time, and Temperature. Nutrients. 2019 Jul; 11(7): 1485.
Post-Exercise Rehydration in Athletes: Effects of Sodium and Carbohydrate in Commercial Hydration Beverages. Nutrients. 2023 Nov 12;15(22):4759.
Optimizing the restoration and maintenance of fluid balance after exercise-induced dehydration. J Appl Physiol 2017 Apr 1;122(4):945-951.
Advanced Nutrition and Human Metabolism, 7th edition. Gropper, SS., Smith, J.L., Carr, T.P. Boston, Massachusetts Cenage Learning, 2017.
American College of Sports Medicine position stand. Exercise and fluid replacement. Medicine & Science in Sports & Exercise 39(2):p 377-390, February 2007
Compositional Aspects of Beverages Designed to Promote Hydration Before, During, and After Exercise: Concepts Revisited. Nutrients. 2023 Dec 20;16(1):17.
Upcoming Articles
Building Balanced Meals and Snacks: Learn how to fuel your body with healthy balanced meals and snacks that meet the high energy and nutrient demands of athletes.